| 流感病毒检测试剂盒(胶体金法) |
|
 |
Diagnos Influenza A+B Antigen Test Kit (Colloidal Gold)
(Cassette)
[INTENDED USE]
The Influenza A+B Antigen Test Kit (Colloidal Gold) is a rapid visual immunoassay for the qualitative, presumptive detection of influenza A and influenza B viral antigen (nucleoprotein) from nasal swab and throat swab specimens from symptomatic patients. The test is intended as an aid in the rapid diagnosis of influenza A and influenza B viral infections.

[MATERIALS SUPPLIED]
1.One pouch contains a test cassette and a desiccant. The desiccant is for storage purposes only and is not used in the test procedures.
2.Sample extraction buffer: 1 bottle (1pc/box), 2 bottles (20pcs/box), 2 bottles (25pcs/box)
3. 1/20/25 Extraction tube(s) and dropper tip(s)
4. 1/20/25 Swab(s)
5. 1 Package insert.
[PRIMARY SAMPLE COLLECTION,HANDING AND STORAGE]
Use the nasopharyngeal swab supplied in the kit.
1. Nasal secretions collection
a) Carefully insert the swab into the nostril of the patient, reaching the surface of posteriornasopharynx. that presents the most secretion under visual inspection.
b) Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
c) Withdraw the swab from the nasal cavity.
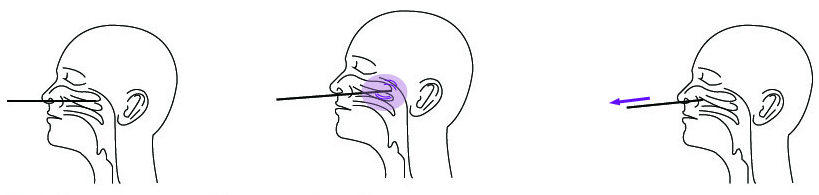
2. Throat secretions collection:
a)Insert the swab completely from the mouth into the throat, centering on the red part of the throat wall and maxillary tonsils.
b) Rub the bilateral throat tonsils and throat wall moderately.
c) Avoid touching the tongue and remove the swab.
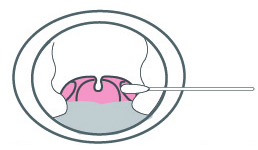
The samples should be treated with the virus sampling solution or the sample extraction solution provided with this kit as soon as possible after collection. And complete the test in 5 minutes.
[TEST PROCEDURE]
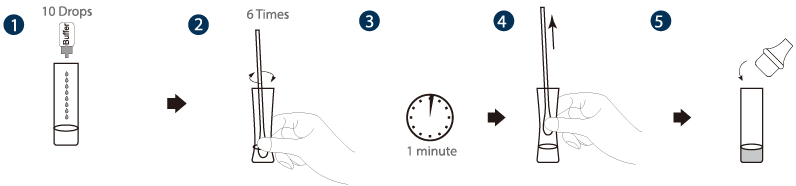
1. Specimen extraction
a) Add 0.5mL (about 10 drops) of the sample extraction buffer into the extraction tube.
b) Insert the swab into the extraction tube which contains 0.5mL of the extraction buffer. Roll the swab at least 6 times while pressing the head against the bottom and side of the extraction tube.
c) Leave the swab in the extraction tube for 1 minute.
d) Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab. The extracted solution will be used as test sample.
e) Fit the dropper tip with filter on top of the extraction tube tightly.
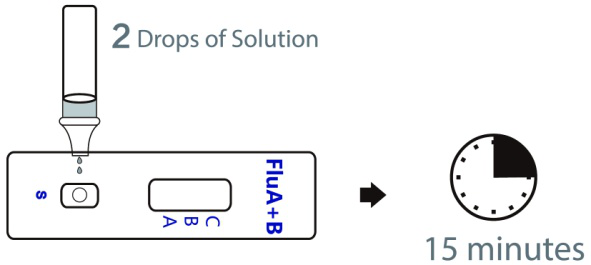
2. Detection operations:
a) Open a pouch containing a test cassette. Place the test cassette on a dry, horizontal work surface.
b) Add 2 drops (about 60μl) of sample solution extract to the sample well respectively of the test cassette.
c) Observe the results showed within 10-15 minutes, and the results showed after 15 minutes have no clinical significance.
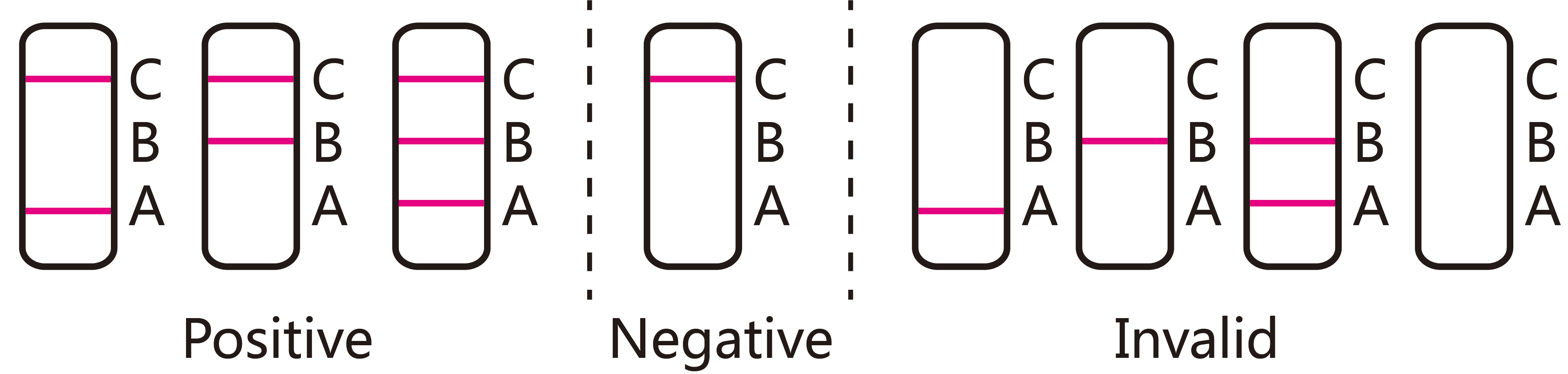
[INTERPRETATION OF RESULTS]
POSITIVE Influenza A: Two distinct colored lines appear. One colored line should be in the control region (C) and another colored line should be in the
Influenza A region (A). The positive result in the Influenza A region indicates that Influenza A antigen was detected in the sample.
POSITIVE Influenza B: Two distinct colored lines appear. One colored line should be in the control region (C) and another colored line should be in the Influenza B region (B). The positive result in the Influenza B region indicates that Influenza B antigen was detected in the sample.
POSITIVE Influenza A and Influenza B: Three distinct colored lines appear. One colored line should be in the control region (C) and two colored line should be in the Influenza A region (A) and Influenza B region (B). The positive results in the Influenza A region and Influenza B region indicates that Influenza A antigen and Influenza B antigen were detected in the sample.
Negative: One colored line appears in the control region (C). No apparent colored line appears in the test line regions (A or B).
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Note:
1. Any shade of color in the test regions (A or B) should be considered positive.
2. Invalid samples should be treated as infectious pollutants, and samples should be collected again.
[CONTACT US]
For more products inquiry , distribution and other professional service, please contact in following ways!
Address: Building 6, Electronic Information Industry Park, 2 Haiyang South Road, Chengnan Subdistrict, Rugao, Rugao, Jiangsu,226000, China.
Tel: +86 513-80116067
Fax: +86 513-85355050
Email:sales@diagnosbio.com
Web:www.diagnosbio.com
|
 |
|
