| Covid-19 Antbody IgG/IgM Test Kit |
|
 |
Diagnos (2019-nCoV) New Coronavirus Antibody Test
(Cassette)
[INTENDED USE]
The (2019-nCoV)New coronavirus Antibody Test is a rapid, qualitative and convenient immunochromatographic in vitro assay for the differential detection of IgM & IgG antibodies to SARS-COV-2 in human serum, plasma or whole blood samples obtained from patient with SARS-COV-2 infection. The device is designed to aid in the determination of recent or previous exposure to SARS-COV-2 virus tracking the status of the disease after SARS-COV-2 infection. It can not be used as a basis for diagnosis and exclusion. This is also not suitable for general population screening.
The test only provides a preliminary result. A positive result does not necessarily mean a current infection, but represents a different stage of the disease after infection. IgM positive or IgM/IgG both positive suggest recent exposure, while IgG positive suggests previous infection, or latent infection. Current infection should be confirmed by Real-Time Reverse Transcriptase (RT-PCR) or viral gene sequencing.
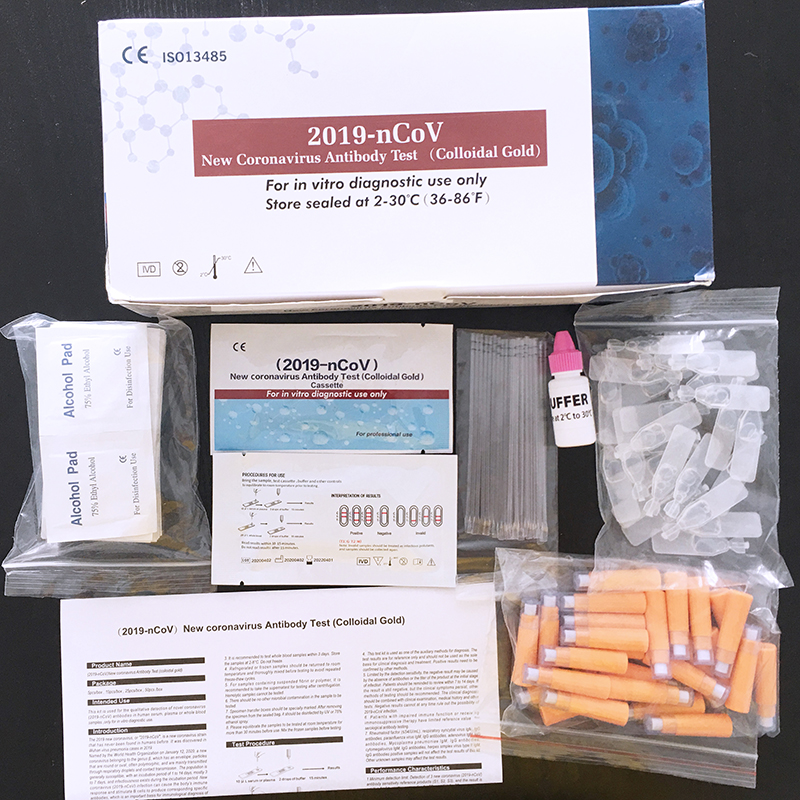
[MATERIALS SUPPLIED]
1. One pouch contains a cassette device and a desiccant. The desiccant is for storage purposes only and is not used in the test procedures.
2. Sample buffer: 1 bottle ×3 ml (5 pcs / box), 1 bottle ×3 ml (10 pcs / box), 1 bottle ×3 ml (25 pcs / box), 1 bottle × 6 ml (50 pcs / box).
3. pipette droppers.
4. Instructions For Use.
|
Product Picture |
Model |
Product size |
PCS/CTN |
CTN size |
G.WN.W |
 |
dnsAb01 |
19*12.3*6.7cm |
1000 |
51.5*40*35.5CM |
10/9.2KGS |
[TEST PROCEDURE]
1.Bring the sample,test cassette and other controls to equilibrate to room temperature prior to testing.
2. Open a pouch containing a test cassette. Place the test cassette on a dry, horizontal work surface.
3. Use a micropipettor or dropper to add 10 μL of serum or plasma samples to the sample well of the test cassette (add 20 μL of whole blood).
4. Add 2 drops of sample buffer (about 70 to 100 μL) to the sample well.
5. Set the timer /clock.
6. Read results within 10-15 minutes. Do not read results after 15 minutes.
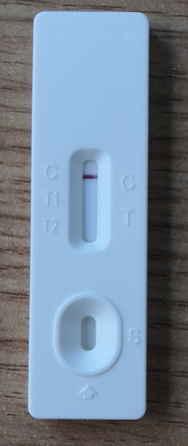
[INTERPRETATION OF RESULTS]
Negative: If only the C band is present, the absence of any pink-rose color in the T1(IgG) and T2(IgM) bands indicates that no anti-2019-nCoV antibodies are detected. The result is negative.
Positive:
1. In addition to the presence of C band, if only T1(IgG) band is developed, the test indicates for the presence of anti-2019-nCoV IgG. The result is positive.
2. In addition to the presence of C band, if only T2(IgM) band is developed, the test indicates for the presence of anti-2019-nCoV IgM. The result is positive.
3. In addition to the presence of C band, both T1(IgG) and T2(IgM) bands are developed, this indicates for the presence of anti-2019-nCoV IgG and IgM. The result is also positive.
Invalid : If no C band is developed, the test is invalid regardless of any pink-rose color in the T1(IgG) and T2(IgM) bands as indicated below. Repeat the test with a new kit. If the problem persists, stop using this lot number immediately and contact your local supplier.
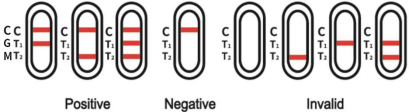
Note: Invalid samples should be treated as infectious pollutants, and samples should be collected again.
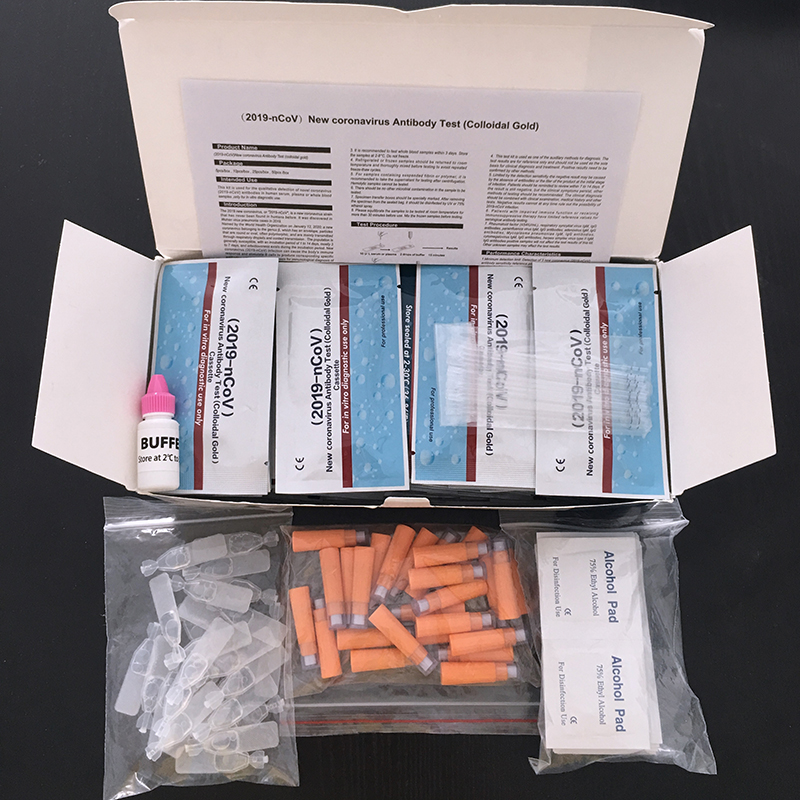
[PACKING]
[CONTACT US]
For more products inquiry , distribution and other professional service, please contact in following ways!
Address: Building 6, Electronic Information Industry Park, 2 Haiyang South Road, Chengnan Subdistrict, Rugao, Rugao, Jiangsu,226000, China.
Tel: +86 513-80116067
Fax: +86 513-85355050
Email:sales@diagnosbio.com
Web:www.diagnosbio.com
|
 |
|
